Six weeks ago, my best friend—someone I’ve known forever—was diagnosed with ovarian cancer. That moment hit me harder than any textbook, study, or medical lecture ever could. As a hematologist who’s spent years wrangling with stubborn immune cells in the lab, facing cancer on this level suddenly became personal. If you’ve ever needed a reason to rethink everything you know about science, watching someone you love battle for their life is it. Today, I want to pull back the curtain—not just on how our immune system works behind the scenes, but on a wave of hope cresting over the cancer research world: CAR NK cell therapy. Come for the science, stay for the surprising possibilities, and maybe leave with a sense that sometimes, even in medicine, the plot twists are the best part.
The Immune System: Unsung Guardian & Reluctant Detective
When we think about what keeps us healthy, most of us picture washing our hands, eating well, or getting enough sleep. But beneath the surface, there’s a silent, tireless force at work: our immune system. As a hematologist and stem cell transplant specialist, I’ve spent years studying this remarkable network. Yet, it wasn’t until ovarian cancer touched my closest friends that the science became deeply personal—and urgent.
Patrolling the Body: The Immune System’s Everyday Heroics
Our immune system is made up of many types of white blood cells, each with a unique job. These cells are constantly on patrol, moving through our blood and tissues, always on the lookout for threats. Most people know that the immune system protects us from infections—bacteria, viruses, and other germs. But its responsibilities go much further.
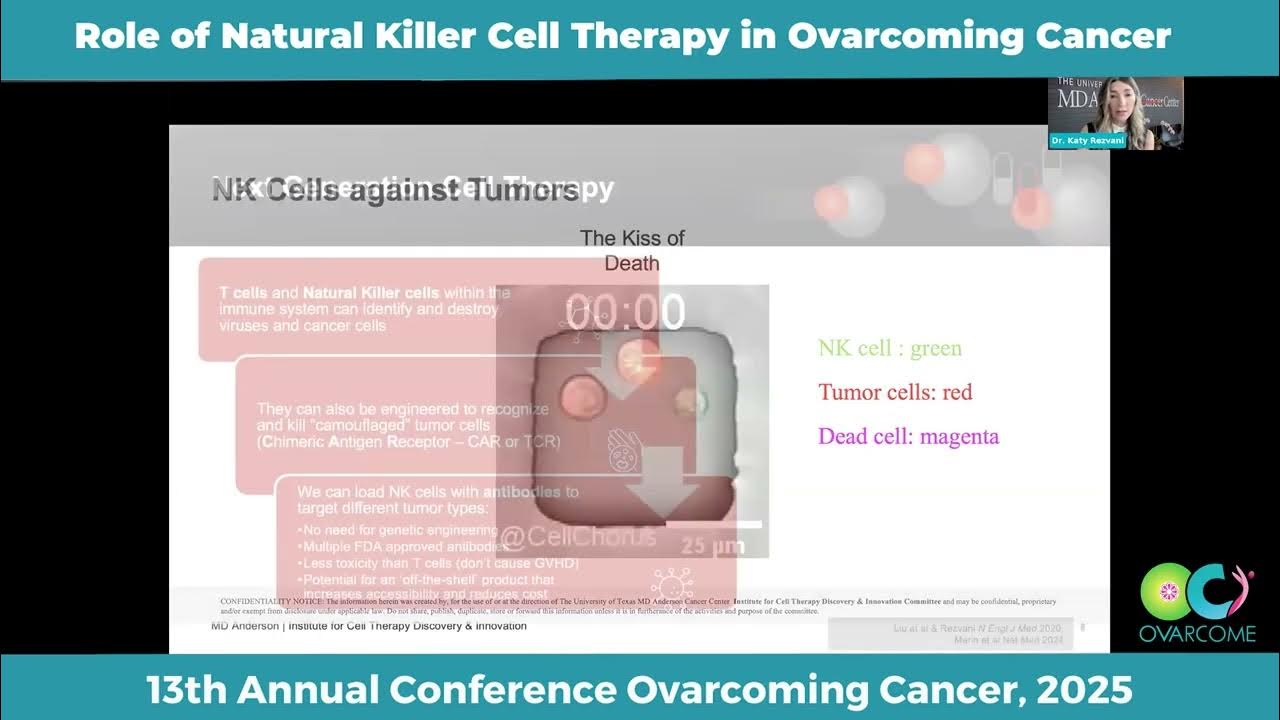
“Our immune system is also very powerful in that it continuously patrols our body to look for abnormal cells for transformed cells such as cancer cells.”
This means our immune system isn’t just a shield against outside invaders. It’s also a detective, searching for internal threats—cells that have gone rogue. These can include cells that have mutated and started to grow out of control, like cancer cells. The immune system’s job is to spot these abnormal cells early and destroy them before they can cause harm.
Cancer’s Survival Trick: Molecular Camouflage
Unfortunately, cancer is a master of disguise. Ovarian cancer, in particular, is known for its ability to hide from the immune system. Cancer cells can change the proteins on their surface, making them look “normal” to immune cells. This molecular camouflage allows them to slip past the body’s defenses and continue growing.
What makes ovarian cancer especially tricky is its heterogeneity—not all tumors look or behave the same. This diversity means that a one-size-fits-all approach often doesn’t work. The immune system may recognize and attack one type of ovarian cancer cell but miss another that looks slightly different. This is one reason why ovarian cancer immunotherapy is both challenging and so urgently needed.
Immunotherapy: Recruiting the Body’s Natural Defenses
Immunotherapy for ovarian cancer is a field that tries to tip the balance back in our favor. Instead of relying only on surgery, chemotherapy, or radiation, immunotherapy aims to retrain the immune system to recognize and destroy cancer cells—even those that are hiding in plain sight.
There are different types of immunotherapy. Some use drugs to “wake up” the immune system. Others, like natural killer cells cancer treatment, use special immune cells that are naturally good at finding and killing abnormal cells. Natural killer (NK) cells are a part of our innate immune system. They don’t need to recognize a specific target; instead, they sense when something isn’t right and act quickly. Scientists are now learning how to harness and enhance these cells to seek out and destroy ovarian cancer cells, even those that have tried to hide.
- Checkpoint inhibitors: Drugs that remove the “brakes” from immune cells, allowing them to attack cancer.
- Adoptive cell therapy: Giving patients immune cells (like NK cells) that have been trained or engineered to target cancer.
- Cancer vaccines: Teaching the immune system to recognize cancer-specific markers.
When Science Becomes Personal
I’ve always been fascinated by the immune system’s detective work. But when my best friend was diagnosed with ovarian cancer just six weeks ago, the stakes changed. Suddenly, the abstract became real. The need for better, smarter ovarian cancer immunotherapy was no longer just a research goal—it was a lifeline for someone I love.
Watching a loved one face ovarian cancer brings home how clever, and how cruel, this disease can be. It also makes me more determined than ever to help the immune system do what it does best: protect us, even when the enemy is hiding in plain sight.
The Unexpected Heroes: Reimagining Cancer Therapy With Off-the-Shelf CAR NK Cells
When I first heard about CAR T cell therapy, it sounded like science fiction. The idea is simple but powerful: take a patient’s own immune cells, train them in a lab to recognize cancer, and send them back into the body as “living drugs.” These cells are armed with a special GPS—called a chimeric antigen receptor (CAR)—that helps them hunt down cancer cells hiding in plain sight. It’s a breakthrough, especially for blood cancers. But as I learned on my own journey with ovarian cancer, this innovation comes with real-world limits.
Traditional CAR T Cell Therapy: Groundbreaking but Out of Reach
CAR T cell therapy is a marvel of modern medicine, but it’s also a logistical and financial challenge. Each dose is custom-made from the patient’s own T cells. Manufacturing takes weeks, and the price tag is staggering—around $500,000 per dose. Even more concerning, only about 20% of eligible patients can actually access this therapy. The side effects can be severe, too, including high fevers, dangerously low blood pressure, and neurological issues. In some cases, patients end up in intensive care. These hurdles mean that, despite its promise, CAR T cell therapy remains out of reach for most people with cancer, especially those with solid tumors like ovarian cancer.
Eureka Moment: Off-the-Shelf Immunotherapy
Researchers began asking a simple but game-changing question: What if we could use donor immune cells instead of making a custom product for every patient? Imagine a therapy that’s ready to go, like grabbing a medication off the pharmacy shelf. This would make treatment faster, more scalable, and potentially much more affordable.
Meet the Natural Killer Cells: Nature’s Cancer Hunters
This is where natural killer (NK) cells enter the story. Unlike T cells, NK cells are the immune system’s first responders. They’re naturally good at recognizing and destroying both virus-infected and cancerous cells. The real surprise? NK cells can be safely transferred from one person to another without causing dangerous immune reactions like graft-versus-host disease. This makes them ideal for off-the-shelf immunotherapy.
Umbilical Cord Blood: An Unexpected Treasure Trove
The next breakthrough came from an unexpected source: umbilical cord blood. Usually discarded after birth, cord blood is rich in healthy, potent NK cells. At MD Anderson, researchers established a cord blood bank and discovered that a single cord blood unit could produce over 100 doses of CAR-engineered NK cells. These umbilical cord blood NK cells can be engineered with a CAR, frozen, and stored—ready to treat patients at a moment’s notice.
| Therapy Type | Source | Cost per Dose | Production Time | Major Risks |
|---|---|---|---|---|
| CAR T Cell | Patient’s T cells | $500,000 | Weeks | Cytokine storm, neurotoxicity, ICU care |
| CAR NK Cell | Cord blood NK cells | $600–$1,000 | Ready off-the-shelf | Minimal, no graft-versus-host disease |
Innovations in Cancer Cell Therapy: Safety, Speed, and Scale
The advantages of CAR NK cell therapy are striking:
- Affordability: Each dose costs less than $1,000, compared to half a million for CAR T cells.
- Accessibility: Off-the-shelf immunotherapy means no waiting weeks for manufacturing—treatment can start almost immediately.
- Safety: Cord blood NK cells don’t trigger dangerous immune reactions, making therapy safer and easier to deliver, even outside major cancer centers.
- Scalability: One cord blood unit can treat over 100 patients, making this approach truly scalable.
Seeing NK Cells in Action: The Wild Card
One of the most unforgettable moments in my journey was watching a video of a single NK cell under the microscope. It would glide up to a cancer cell, “kiss” it, and move on—leaving the cancer cell to die. Then it would repeat this with another, and another. It was both creepy and awe-inspiring to see these tiny, natural-born killers at work.
“And our results were unprecedented in that of the 42 patients that we treated, 93% had evidence of their tumor shrinking and in 2/3 of the patients the cancer was completely gone and that’s what we call complete remission and we didn’t see any toxicities.”
This is the promise of CAR NK cell therapy: a safe, scalable, and affordable innovation in cancer cell therapy that could finally bring the power of off-the-shelf immunotherapy to more people, including those with ovarian cancer, no matter where they live.
Beyond Blood Cancers: Solid Tumors, New Targets, and the Next Chapter
When I first heard about CAR NK cell therapy, it was always in the context of blood cancers—leukemias, lymphomas, and other diseases where the immune system’s natural killer (NK) cells could be engineered to hunt down cancer cells. The early clinical trials, like the one launched at MD Anderson in 2016, focused on B cell malignancies using cord blood-derived CAR NK cells targeting CD19. The results were nothing short of astonishing: patients who had failed multiple rounds of chemotherapy, stem cell transplants, and even CAR T cell therapy saw their tumors shrink or disappear. In one study, 93% of 42 lymphoma patients had tumor shrinkage, and two-thirds achieved full remission. Even more remarkable, these therapies came without the severe side effects that often haunt other immunotherapies—no high fevers, no dangerous drops in blood pressure, and no ICU stays. The manufacturing cost was reported at just $600 per dose, and 250 patient doses were produced in only two manufacturing runs.
But as someone living with ovarian cancer, I always wondered: could this kind of immunotherapy work for solid tumors, too? Solid tumor immunotherapy, especially for cancers like ovarian, kidney, or breast, is notoriously tricky. These tumors are experts at hiding from the immune system, building protective barriers, and creating environments that suppress immune attacks. For years, immunotherapy for ovarian cancer felt like a distant dream—one that always seemed just out of reach.
That’s why the recent shift in research is so exciting. As one of the lead scientists put it,
“So now we really have started focusing on solid tumors.”
The move from blood cancers to solid tumors marks a new chapter, not just for research, but for patients like me. The key lies in finding new molecular targets—unique “beacons” on tumor cells that can be recognized by engineered CAR NK cells. After CD19 and CD30, researchers have identified targets like CD70 and TROP2, which are found on a range of solid tumors, including ovarian and kidney cancers. This kind of precision engineering means that CAR NK cell therapy is no longer limited to blood cancers; it’s being tailored to hunt down the unique signatures of solid tumors.
The clinical trials for these new targets have already begun. What’s truly groundbreaking is that these therapies are being delivered in outpatient settings. Patients receive a gentle, three-day course of chemotherapy, then a simple infusion of thawed CAR NK cells at the bedside—no need for intensive hospital stays. Even at micro-dose levels, patients with aggressive, treatment-resistant cancers are seeing their tumors shrink or vanish. The safety profile remains strong, with no severe toxicities or graft-versus-host disease, even when using mismatched cord blood units. For patients who have exhausted every other option, this is nothing short of a lifeline.
For me, participating in a clinical trial for immunotherapy for ovarian cancer felt both surreal and hopeful. The process was efficient and, compared to traditional treatments, far less grueling. I felt like I was part of something bigger—a movement that was rewriting the rules of cancer care. The science behind CAR NK cell therapy sometimes feels like science fiction: using cells from donated umbilical cords, engineering them with a kind of GPS to seek out cancer, and delivering them in a way that’s accessible and affordable. But this isn’t a story about gadgets or imaginary villains; it’s about real lives, real hope, and a future where solid tumor immunotherapy is within reach for more people than ever before.
As the research community continues to push boundaries, the paradigm is shifting. Clinical trials for blood cancers paved the way, but now, with new targets and outpatient protocols, CAR NK cell therapy is poised to change the landscape for solid tumors like ovarian cancer. The next chapter is being written right now—in labs, in clinics, and in the lives of patients who finally have reason to hope. For anyone facing a diagnosis that once seemed hopeless, this is the promise of a new beginning, and I am grateful to be part of it.
Leave a Reply